Have you ever wondered how soap works? Do we need bubbles? How does soap clean?

I was sure I knew the answer, but I asked Seth Klein, one of the Chemistry teachers in the school district where I work. Seth said, Soap contains complex particles that allows it to interact with both polar (water) and non polar (dirt and grease) molecules. Soap particles can therefore orient themselves into micelles, which are little circles, that encapsulate the grease/dirt and allow it to be removed.
Let’s break this down.
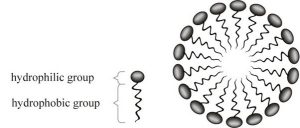
A polar molecule is hydrophilic (will mix with water, we’ll say water-loving)
A non polar molecule is hydrophobic (doesn’t mix with water, we’ll say oil-loving)
Oil and water don’t mix.
Soap has a polar end and a non polar end.
Most dirt contains oil and grease.
Soap can mix with water and oil, because it has a polar end (mixes with water) and a non polar end (mixes with oil)
The soap molecules form a sphere called a micelle when mixed with water and dirt.
The water-loving part of the soap molecule attaches to the water, and the oil-loving part attaches to the dirt.
The dirt gets is in the middle of the micelle, and gets rinsed away.
I found a video if you’re more of a visual person. This one’s pretty good too.
A micelle is not a bubble. Bubbles are thin spheres of liquid enclosing air or another gas. The amount of bubbles, or lather, has no bearing on how much cleaning is going on.
A great big thank you to Seth for his help. I think I’ll leave a few bars on his desk to show my gratitude.
Yours in Gratitude,
Angela

Sorry Ang. You lost me at “dirty.” Chemistry was NOT my subject in school. All I know is your soaps make me happy, I get clean and the ingredients are safe!
You have me cracking up Gay! But I am glad my soaps make you happy. 🙂